AFFORDABLE & FLEXIBLE
Built to Work with Any Textbook
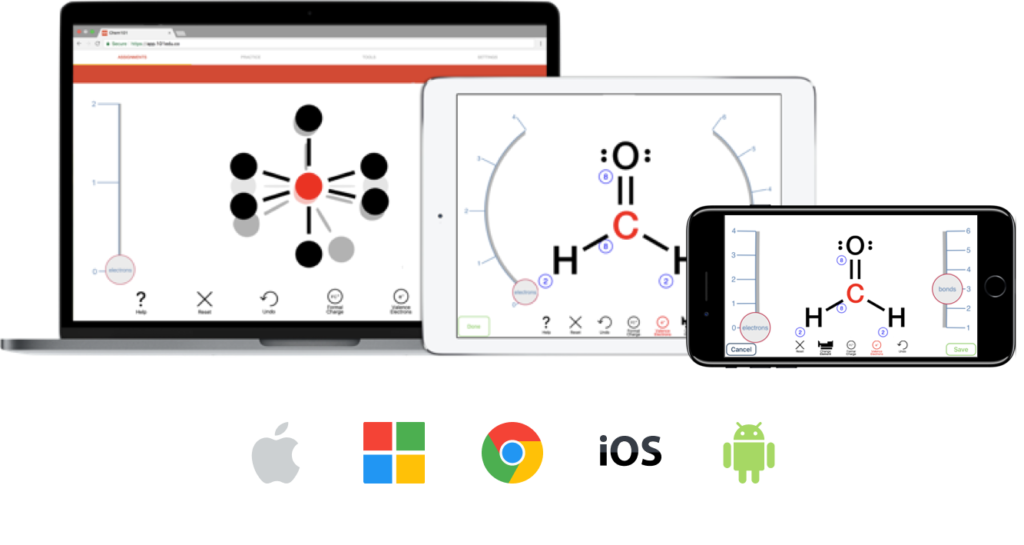
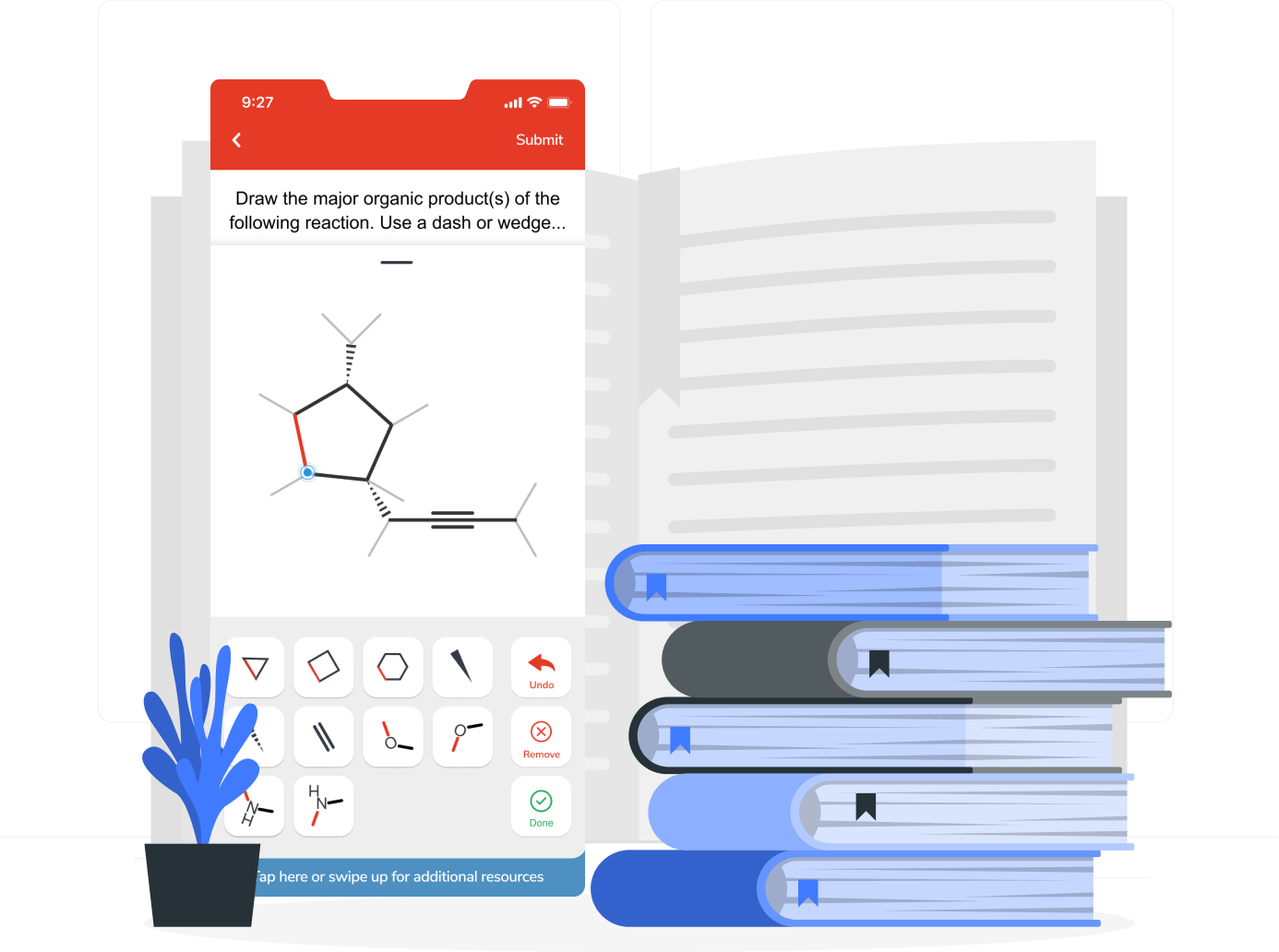
Aktiv Chemistry’s textbook independent approach means instructors have more flexibility for their courses. Build assignments that align to any major publisher textbook or increasingly popular OER options.
For General Chemistry courses, Aktiv Chemistry also offers seamless integration with the OpenStax Chemistry textbooks.