Issue #2
Welcome to the second edition of our monthly digest Equilibrium!
From mnemonics to misconceptions about chemical equilibrium to IMF! We hope you enjoy these practical insights from creative instructors and educational researchers.

Justin Weinberg
Co-founder & CEO
Sign up to receive this monthly newsletter
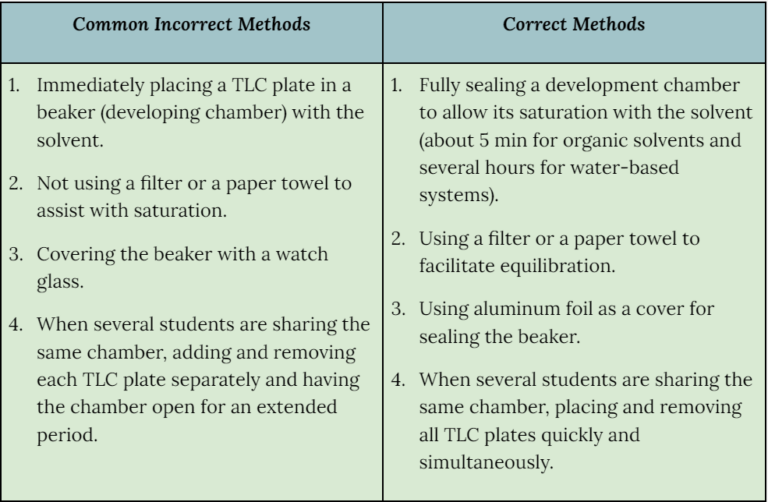
Do you teach the proper TLC technique?
It is essential that beginner students see that their predictions about chemical behavior are supported in laboratory experiments. This pattern of lecture-to-lab reinforces knowledge and improves confidence. If lab experiment results are unclear or not reproducible, there is a danger of students concluding that science is “iffy” and unreliable. Advanced students may benefit from opportunities to investigate and discuss multiple variables and the complexity of a chemical experiment, but early learners need consistent, dependable experiments until they develop a solid understanding of the scientific method.
Thin Layer Chromatography is a common laboratory technique used widely in Introductory, General Chemistry, and GOB courses due to its easily obtainable and clear results. However, incorrect methods may lead to inconsistent outcomes, so it is important to ensure students adhere to the accurate methodology. Here are the suggestions from the scientist from Teledyne Isco Inc., a company that manufactures a wide range of products for professionals working in the field and in laboratory work.
Mnemonics for Writing Net Ionic Equations
Chemistry faculty worldwide have been trying various pedagogical techniques to help students make sense of chemistry, retain and transfer knowledge. This is especially challenging for those who teach students who are non-chemistry majors. These students often have preconceived notions about chemistry. For example, a 2018 survey of 136 allied health students confirmed that they often see chemistry as the most challenging subject among prereq science courses.
One way to help such students learn is to develop shortcuts for memorizing steps of complex problem solving, such as diagrams and mnemonics. For example, Dr. Angela L. Mahaffey from the Loyola University of Chicago uses two mnemonics to help students with writing and interpreting net ionic equations:
- “Take a MINute” mnemonic reminds students to pause and assess the step-by-step process for writing a net ionic
equation: Molecular equation (write and balance an equation) – Ionic equation (identify and separate ions) – Net ionic equation (identify and remove spectator ions) - “Don’t touch the LeGS!” mnemonic helps to remind students to focus only on the aqueous compounds in writing net ionic equations, and not liquid (L), solid (S), or gaseous (G) compounds.
Note that although mnemonics are great for simple memorizations of steps or facts, one must be careful not to rely too heavily on them when a more intricate analytical thinking process is involved.
Making Intermolecular Forces (IMF) visible
Ask any introductory chemistry student about the gas bubbles that appear on the surface of water when it boils. At least several people in the class would say that they are oxygen or hydrogen bubbles, because “that is what water is made of.” Ask them what holds water molecules together in an ice cube and chances are you’ll hear ‘crickets.’
Intermolecular forces (IMF) concept is hard for many beginner students, but the importance of it for explaining physical properties of matter cannot be underestimated. Here are some of the prompts you can try in your class to help students understand IMF.
- Suggest using this free simulation to discover which molecules, with low or high polarity, have stronger IMF.
- Ask students to explain why polar and nonpolar substances have very different boiling points using this free simulation.
- Task students to predict the IMF between molecules of hydrofluoric acid using this free simulation.
- Ask students to apply the IMF concept to explain why vinaigrette salad dressing has two distinct layers of liquid. Also, ask them to hypothesize why these layers mix temporarily if you vigorously shake it.
- Provide students with small samples of isopropyl alcohol, water and glycerine. Ask them to place one drop of each on a paper towel and observe which liquid evaporates the fastest. Then prompt them to explain their observations using the IMF forces concept.
- As a continuation of the previous demonstration, ask students to sprinkle pepper on the top of the same three liquids (isopropyl alcohol, water and glycerine), each in its own small beaker, and observe what happens. Then provide a set of tweezers and plastic covered paper clips. For each liquid that supports pepper on its surface, ask students to pick up one plastic covered paper clip with tweezers and gently place it on top of the liquid surface (horizontally). They will see that water cannot hold even pepper, and only glycerine can hold a paper clip. Ask students to explain what happens on the intermolecular level for this to happen.
Preventing/Correcting misconceptions about chemical equilibrium
Are you finding yourself constantly correcting students’ intuitive misconceptions by saying?
- “No. Many chemical reactions are reversible.”
- “No. Reactions do not stop at equilibrium.”
- “No. Equilibrium constant doesn’t increase with the addition of the reactant.”
- “No. Equilibrium doesn’t mean concentrations of reactants and products are equal”, etc.
Try the following hands-on activities from the Royal Society of Chemistry:
- Activity 1: Students observe the equilibrium adjustment in this experiment as the carbonated water solution becomes less acidic.
- Activity 2: Students observe the effect of concentration, catalyst and temperature on the equilibrium system: Fe3+(aq) + SCN–(aq) → [FeSCN]2+(aq)
- Activity 3: Students observe how the equilibrium between two different colored cobalt species is disturbed and use Le Chatelier’s principle to predict a color change.
- Activity 4: Students determine the equilibrium constant for the reaction between methanol, CH3OH, and ethanoic acid, CH3COOH, to form the ester methyl ethanoate, CH3COOCH3, and water.
- Activity 5: The effect of temperature on the equilibrium between the two cobalt species: [CoCl4]2-(aq) + 6H2O(l) ⇌ [Co(H2O)6]2+(aq) + 4Cl–(aq)
What did you think of the newsletter?







